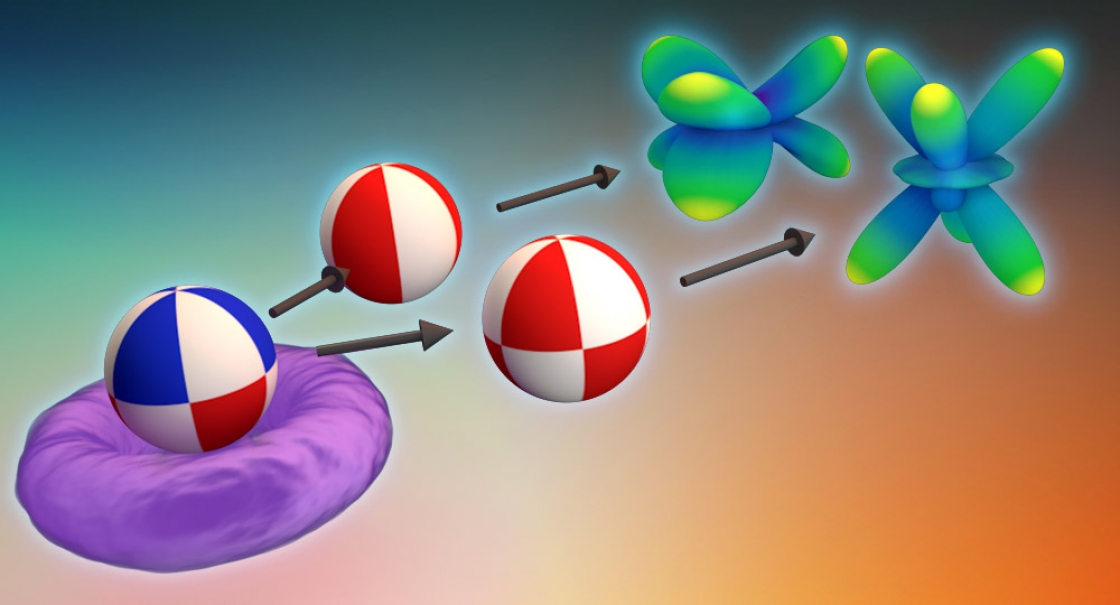
Atoms are busy objects. Electrons whiz around the nucleus of the atom in attoseconds—quintillionths of a second. Those electrons can be orbiting farther out from the nucleus in an excited state, close to the nucleus in the lowest energy level called a ground state, or in a superposition—in two or more energy levels at once.
During ionization, some of those electrons will fly away from the atom. The direction and path those electrons take can tell scientists a lot about the state the atom was in before—ground state, excited state, or superposition.
“We want tools that can identify if it’s this particular state or that particular state. That’s important in the ionization process,” said Joel Venzke, a graduate student at JILA. “[We] would like to know exactly what that superposition is, and what is the relative phase between these two states…we want to follow this motion and be able to write down the wave function at a certain point in time.”
Venzke has developed a set of tools to do this using the time-dependent Schrödinger equation and ultrashort laser pulses to capture the path of the electron and the state of the atom during ionization. The team’s results were published in Nature Scientific Reports.
Asymmetry in motion
The way an electron leaves an atom during ionization can tell scientists the state of the atom at the time. JILA’s researchers have used two key tools to track the electron’s escape: attosecond (10-18 second) laser pulses and numerical solutions to the Schrödinger equation—a differential equation which describes the evolution of the wave function of a quantum system in time. In this scheme, the electron is stripped from the atom by the attosecond laser pulse, and the rapid laser pulses take snapshot images of the atomic state and the ionization process, by capturing the directions in which the electrons fly.
“If it was in the ground state, the electron wave function is essentially distributed in this ball [around the nucleus]. If it’s in its excited state, it’s in a donut. If it’s in the superposition state, it is moving around,” said JILA Fellow Andreas Becker.
“We're interested in how does that shape break down from being either this perfect donut or perfect ball into this kind of asymmetric shape [of the ionized electrons],” Venzke added.
Using the Schrödinger equation, scientists can determine the electrons’ “flight paths” from the symmetrical or asymmetrical shapes that illustrate the directions in which the electrons are leaving the atom. Venzke, with some guidance from JILA Fellows Becker and Agnieszka Jaron-Becker, took the lead and developed generalized asymmetry parameters (GAPs) to quantify these uneven, asymmetrical distributions—which tells them valuable new information about how those electrons left the atom.
“The asymmetry tells us something about which of the possible pathways is dominant, or if they are interfering,” Becker said. “It tells us how the electron was actually removed from the atom and which pathway did it take, or was there more than one pathway involved in it.”
Studying and quantifying atoms in their excited or superposition states is really important in physics, Venzke said. And attosecond laser pulses are useful to capture and follow the motion of the electrons in these states. With this insight, scientists can better understand what happens during interactions between atoms—perhaps even capturing videos of those interactions—or whether electrons are exchanging energy and what energy levels they are occupying.
More importantly, if scientists can see what happens in these interactions, there’s the possibility that they can manipulate those interactions—although that’s still some way off, Becker added.
“It’s a small piece of the puzzle,” Becker said. “First, we have to understand. Then, once we understand, we can try to control it…there are many steps in between. But it’s part of the process.”
This study was published in Nature Scientific Reports on September 30, 2020. It was supported by a National Science Foundation Physics Frontier Center grant and the U.S. Department of Energy, Division of Chemical Sciences, Atomic, Molecular and Optical Sciences Program.
Written by Rebecca Jacobson


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.